5 Years of OIG Results
Summary:
The COVID-19 pandemic has presented a monumental challenge to public health experts, governments, and private organizations alike. To date, there are currently 20 different coronavirus vaccines available or in development that have been approved for use by at least one regulatory body around the world. Each vaccine is unique in its composition and development process, offering varied levels of efficacy and safety. Sounds great, but the financial burden of coordination with providers and hospitals particularly the government has led to a huge increase in healthcare spending even during a downturn in COVID diagnoses and deaths.
Covid 19 Vaccine Costs
Covid-19 Vaccine History
Vaccines developed to prevent the spread of Covid-19 have quickly become some of the most sought-after pharmaceutical products in history. This can be largely attributed to the sheer scale of need created by the pandemic, which spurred a massive effort from drugmakers around the world to produce safe and effective treatments on an accelerated timeline. The demand for these medicines has received significant support from governments and philanthropic organizations, who have collectively earmarked billions of dollars for vaccine procurement and distribution.
Pfizer is expected to post $101.3 Billion or more in earnings from their COVID vaccines, while Johnson and Johnson could report similar earnings as they grew from 2018 ($53.7 billion) to 2019 ($82.6 billion). Most notable is the oral COVID drug Paxlovid which likely will post $30 billion or more in 2022 was found to successfully reduce hospitalization and/or death in patients by up to 89%! Paxlovid is used in combination with the HIV drug Ritonavir.
Why Does this Matter to You as a Payer or Provider?
Pfizer has become a huge influence in determining U.S. Healthcare Policy going forward. Since they became the #1 creator of COVID-19 vaccines they have been able to successfully raise their prices to the Biden Administration’s budget on public health. The Biden Administration in late 2022 bought 105 million doses for $3.2 Billion, which equates to an increase from $19.50 a dose rate to a $30.47 dose rate. Yes, folks, the cost of the vaccine is going up. But why?
Well, when you have an epidemic and the demand is high, the price can be lower or medium based on the emergency status of both patients and the staffing of hospitals, but COVID rates of infection are on the decline.
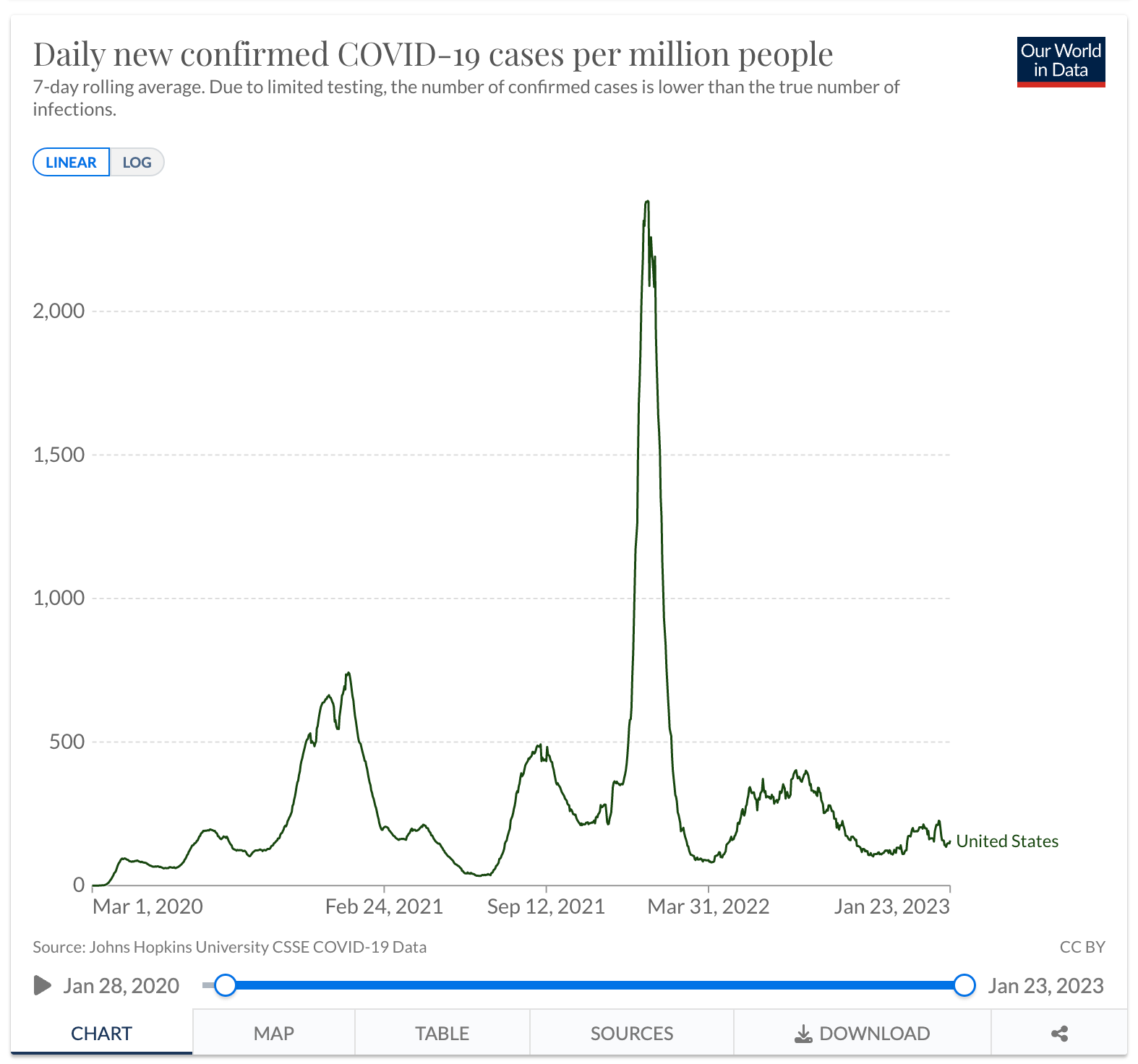
The graph below shows the new cases per day in the USA. You can see that the spikes of COVID cases began with a slow uptick in Q1 2020, then grew dramatically from Q4 2020 - Q2 2021, while the biggest growth was seen in Q1 of 2022. Now that daily COVID cases have decreased and there are more treatment options for patients, the demand for treatment has decreased leading Big Pharma to raise the price of the drugs in an attempt to continue its profits.
COVID Death Rates
There has to come a time when we as a general public need to establish COVID-19 as a treatable and not a worldly pandemic mindset. In just a single year, COVID-19 deaths have fallen from 2,179 deaths on Jan 24, 2022; to 270 deaths on Jan 24, 2023. We are better informed and have more treatment options, yet the Federal Government continues to pay more for the vaccines and boosters… PCG is not standing on one side of the vaccination or non-vaccination fence, we are just stating that with death rates declining, shouldn’t the US Healthcare System and the White House be demanding that the Vaccine price decline?
COVID-19 Statistics vs. Influenza (Flu)
From 2011 - 2019, the USA averaged 20 million to 40 million flu cases, 25,000 - 50,000 deaths per year, 10-18 million medical visits, and over 400,000 hospital visits for the flu. However, since COVID-19 came into our lives we had approximately 9 million flu cases from 2021-2022 instead of 20-40 million. We’ve had only 5,000 to 7,500 flu deaths. As a provider and a payer, we need to be able to question the legitimacy of a COVID diagnosis which endures far more future costs at this time than a flu diagnosis.
Per both John Hopkins and the Mayo Clinic; over 80% of deaths result from those 65 or older, and those who have the hardest time battling COVID and its possible long-lasting effects are those with pre-existing diseases of the lung, heart, brain, cancer, kidneys, down syndrome, and diabetes and/or obesity. So wouldn’t it make sense that COVID-19 campaigns be more focused on educating these patient populations? Wouldn’t it make more sense to spend more time and bill for more time with patients who fall under these conditions and see if COVID-19 treatments and preventative care are going to help Americans? We don’t know, that’s why we’re asking you as providers, payers, and healthcare professionals…
COVID Billing Information
Who Administers Vaccines?
Per CMS and the CDC, only registered providers with the CDC COVID-19 Vaccination Program can legally possess, distribute, deliver, administer, receive shipments of, handle, and administer COVID-19 vaccines in America. What’s the problem you ask? We have a National Shortage of Nurses, Mid-Levels, and Pharmacists available… The multi-dose and boosters need the administration to be logged and planned. We don’t know the perfect solution for COVID-19 vaccination and treatment, but we can tell you that other vaccines are administered faster and easier and have far less administrative and financial costs to healthcare than COVID-19.
Who Pays for Vaccines and Administration?
Per CMS (Jan 2023), patients no matter in-network or uninsured will never be required to pay a copay for a COVID-19 vaccine or its administration and they don’t require a provider’s order or supervision. While the Patient doesn’t pay your clinic may bill Medicare for administering COVID-19 vaccines to Medicare patients. You can bill single claims or a roster claim for multiple patients at a time, just be sure to document your processes with POS (point of service) codes. If you’re unfamiliar with or struggling with this our IVECoder and Virtual AuthTech solutions help providers and payers with this information.
Temporary POS and NPIs
Again, When administering the COVID-19 vaccine, healthcare providers must provide it at no cost to the patient. This means that Medicare and other insurers should not be billed for the vaccine, nor should patients be asked to submit a claim. There is an exception if the provider receives government funding for administering the vaccine, in which case they can still submit a claim to Medicare. Additionally, providers should include ICD-10 diagnosis code Z23 on their claims and may need to include modifier CR if administering at a temporary location rather than their own practice locations. Providers should also ensure any National Provider Identifiers (NPIs) tied to multiple Provider Transaction Access Numbers (PTANs) have the correct taxonomy code so they can assign the correct PTAN. By submitting claims to a payer correctly we can avoid COVID waste, abuse, and fraud, as well as lost earnings to providers due to denials and appeals.
Listing of All COVID Vaccines
1. Pfizer-BioNTech COVID-19 Vaccine (BNT162b2): Developed by Pfizer and BioNTech, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.fda.gov/media/144414/download
Citation: U.S Food and Drug Administration. “Pfizer’s Emergency Use Authorization for BNT162b2 mRNA COVID-19 Vaccine” December 11, 2020,
2. Moderna COVID- 19 Vaccine (mRNA -1273): Developed by Moderna, Inc., this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.modernatx.com/covidvaccineeauth
Citation: Moderna Press Release “Moderna Announces FDA Grant of Emergency Use Authorization for the Preventive use of its Messenger RNA (mRNA) 1273 SARS CoV 2 Vaccine against Covid 19 in Individuals 18 Years or Older” December 18th 2020
3 Johnson & Johnson Janssen COVID – 19 Vaccine Ad26 .COV2 -S): Developed by Johnson & Johnson Janssen, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.jnj.com/johnson-johnson-announces-euas-for-one-dose-covid 19vaccine
Citation: Johnson & Johnson Press Release “Johnson & Johnson Announces EUAs for One-Dose COVID-19 Vaccine” March 10, 2021,
4. AstraZeneca COVID - 19 Vaccine (AZD1222): Developed by AstraZeneca and Oxford University, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.astrazeneca.com/media-centre/press-releases/2021/euas-for-oxfordastrazeneca-vaccine.html
Citation: AstraZeneca Press Release “EUAs for Oxford / AstraZeneca Vaccine” February 23, 2021.
5. Novavax COVID-19 Vaccine (NVX -CoV2373): Developed by Novavax, Inc., this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.novavax.com/news-releases/novavax-announces-u-s-fda-grants-emergency-use-authorization-covid 19vaccine
Citation: Novavax Press Release “Novavax Announces U.S. FDA Grants Emergency Use Authorization for COVID -19 Vaccine” May 10, 2021.
6. Sinovac COVID -19 Vaccine (CoronaVac): Developed by Chinese company Sinovac Biotech Ltd., this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.sinovac.com/en/
Citation: Sinovac Biotech Ltd. “Sinovac CoronaVac Vaccine” May 14, 2021.
7. Sputnik V COVID-19 Vaccine (Gam -COVID -Vac): Developed by Russian developers Gamaleya Research Institute of Epidemiology and Microbiology, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://sputnikvaccine.com/en/
Citation: Russian Direct Investment Fund “RDIF and Gamaleya Research Institute of Epidemiology and Microbiology Announce Joint Development of Vaccine Against Covid 19” August 11, 2020.
8. CanSino Biologics COVID-19 Vaccine (Adenovirus type 5 Vector): Developed by Chinese company CanSino Biologics Inc., this vaccine is used to prevent Coronavirus disease (COVID-19). Link: http://www.cansinobiologics.com/
Citation: CanSino Biologics Press Release “CanSinoBIO Announces Clinical Trial Results of its Adenovirus Type 5 Vector-Based COVID 19 Vaccine Candidate” March 24, 2020.
9. Sinopharm COVID-19 Vaccine (BBIBP-CorV): Developed by Chinese company Sinopharm, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.sinopharm.com/
Citation: Sinopharm Press Release “Sinopharm Receives Emergency Use Authorization for its COVID 19 Vaccine in China” July 20, 2020.
10. Bharat Biotech COVID-19 Vaccine (Covaxin): Developed by Indian company Bharat Biotech, this vaccine is used to prevent Coronavirus disease (COVID- 19). Link: https://bharatbiotech.com/en/
Citation: Bharat Biotech Press Release “Bharat Biotech Announces Emergency Use Authorization for Covaxin, India’s First Indigenous COVID-19 Vaccine” January 7, 2021.
11. Curevac COVID-19 Vaccine (CVnCoV): Developed by German biopharmaceutical company CureVac, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.curevac.com/
Citation: CureVac Press Release “CureVac Receives European Commission Authorization for its COVID 19 Vaccine” January 11, 2021.
12. Janssen-Cilag COVID-19 Vaccine (Ad26.COV2-S): Developed by Johnson & Johnson subsidiary Janssen, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.janssen.com/
Citation: Johnson & Johnson Press Release “Johnson & Johnson Announces Emergency Use Authorization of Janssen COVID 19 Vaccine Ad26.COV2-S” March 8, 2021.
13. Moderna COVID-19 Vaccine (mRNA-1273): Developed by Moderna, Inc., this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.modernatx.com/
Citation: Moderna Press Release “Moderna Announces U.S. FDA Grants Emergency Use Authorization for mRNA-1273” December 18, 2020.
14. Pfizer-BioNTech COVID-19 Vaccine (BNT162b2): Developed by pharmaceutical giants Pfizer and BioNTech, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.pfizer.com/
Citation: Pfizer Press Release “Pfizer and BioNTech Announce U.S. FDA Grants Emergency Use Authorization for BNT162b2 Vaccine Against COVID-19” December 11, 2020.
15. AZD1222 (formerly ChAdOx1 nCoV-19): Developed by AstraZeneca and Oxford University, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.astrazeneca.com/
Citation: AstraZeneca Press Release “AstraZeneca Announces U.S. FDA Grants Emergency Use Authorization for AZD1222 Vaccine Against COVID-19” December 21, 2020.
16. Novavax COVID-19 Vaccine (NVX‑CoV2373): Developed by Maryland company Novavax Inc., this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.novavax.com/
Citation: Novavax Press Release “Novavax Announces U.S. FDA Grants Emergency Use Authorization for NVX-CoV2373 Vaccine Against COVID-19” January 22, 2021.
17. Sputnik V (Gam-COVID-Vac): Developed by the Russian Gamaleya Institute and distributed by the Russian Direct Investment Fund, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://sputnikvaccine.com/
Citation: RDIF Press Release “Russian Direct Investment Fund Announces U.S. FDA Grants Emergency Use Authorization for Sputnik V Vaccine Against COVID-19” February 2, 2021.
18. Sinovac COVID-19 Vaccine (CoronaVac): Developed by Chinese company Sinovac Biotech Ltd., this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.sinovac.com/
Citation: Sinovac Press Release “Sinovac Biotech Receives Emergency Use Authorization for its COVID-19 Vaccine in China” July 17, 2020.
19. Valneva COVID-19 Vaccine (VLA2001): Developed by Austrian-French biotechnology company Valneva, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.valneva.com/
Citation: Valneva Press Release “Valneva Announces Positive Regulatory Updates for its COVID 19 Vaccine Candidate VLA2001” February 22, 2021.
20. Gamaleya COVID-19 Vaccine (EpiVacCorona): Developed by the Russian Gamaleya Institute, this vaccine is used to prevent Coronavirus disease (COVID-19). Link: https://www.gamaleya.ru/en/
Citation: Gamaleya Institute Press Release “Gamaleya Institute Announces U.S. FDA Grants Emergency Use Authorization for EpiVacCorona Vaccine Against COVID-19” January 20, 2021.
Biggest Fraud in US History
COVID-19 Fraud, Schemes, and Impact
COVID Fraud has been the single largest industry of fraud in the history of our planet. In April 2022, the Department of Justice charged 21 defendants in nine federal districts across the US for cases that totaled $149 million in COVID-19-related false billings!
On September 20, 2022, the U.S. Attorney announced they were charging 47 Defendants for participating in and defrauding the US Healthcare system, patients, and payers of $250 million in federal funding that was intended to provide meals for children during the Pandemic.
A Florida provider was charged with wire fraud, healthcare fraud, and kickback schemes, and could face up to 82 months in prison for launching a telemedicine encounter program that violated CMS’ regulations on increased access to care for COVID-19 patients.
Why does this matter to you as a provider, taxpayer, health plan or IPA, TPA, or MSO?
Because patients didn’t pay anything, you and we did. We finance the federal government with our taxes, these taxes pay out the federal funding of COVID-19 administration and all its fees.
Treasury Department scams reported by CMS involve patients and providers giving out PHI and even credit card information to enroll them in grants or stimulus packages to qualify for treatment and/or financial support. You can report a scam by visiting here and reporting it:
www.ic3.gov.
How PCG Can Help
It is impossible for any one organization; government or private, to analyze every claim, every diagnosis, and every expense. With a shortage of US capable workers, the solution has to be AI Healthcare Software. Our Virtual Examiner ® engine for Payers allows payers to review up to 3 million claims per night, and when the payer teams come in the morning they have dedicated reports on the claims that don’t follow the rules. For Providers, IVECoder ® allows them to quickly find any type of billing code or diagnosis imaginable thus properly submitting claims correctly or filing appeals faster.
Summary:
As Healthcare Providers, Payers, and Organizations, our first priority is to given world-class care and treatment options and guidance to patients. Our second obligation is to look at what’s going on with patients, claims, and control actions within our control to avoid excess spending and report fraud. Lastly, when we see an abuse of power within healthcare that is affecting either/or patient care or the inflation of healthcare as a whole we need to stand up. If we don’t serve these roles the cost of healthcare will continue to grow, and decrease the standard of care, and the standard of living… for all patients and taxpayers in our amazing country, the United States of America.
References
- CDC timeline reference link
- NCBI findings on impacts to payers
- EY's findings on full impact of payers for Medicaid, Commercial and Medi-Medi
Our History and Credibility in Reporting this Information:
For over 30 years, PCG Software Inc. has been a leader in AI-powered medical coding solutions, helping Health Plans, MSOs, IPAs, TPAs, and Health Systems save millions annually by reducing costs, fraud, waste, abuse, and improving claims and compliance department efficiencies. Our innovative software solutions include Virtual Examiner® for Payers, VEWS™ for Payers and Billing Software integrations, and iVECoder® for clinics.